Page 3 - MINMAT :: CSIR-IMMT News Letter
P. 3
Research Highlights
A study on variation of atmospheric pollutants over Bhubaneswar during imposition of nationwide lockdown
in India for the COVID-19 pandemic
CSIR-IMMT is undertaking research about atmospheric sciences, in
particular for Bhubaneswar and neighbouring areas. During the Covid-19
pandemic also, researchers have undertaken several work related to air
quality. It was observed that the nationwide lockdown in India to flatten the
pandemic COVID-19 curve has resulted in the reduction of anthropogenic
emission sources to a great extent. Their study reports change in air quality
and its impact on the environment during the unique lockdown scenario at
Bhubaneswar, a coastal smart city in east India. The urban air shows a
remarkable reduction in the mean pollutant levels influenced by traffic
emission viz. NO (~ 67 %) and BC (~ 47 %) during lockdown over the pre-
x
lockdown. Comparatively, a lower reduction of CO (~ 14 %) is attributed to
the dominance of natural atmospheric chemical regulation and biogenic
sources in addition to anthropogenic contributions.
Source: Air Qual Atmos Health, 14, 97-108 (2021); Team Lead by Dr. Trupti Das and Dr. R Boopathy
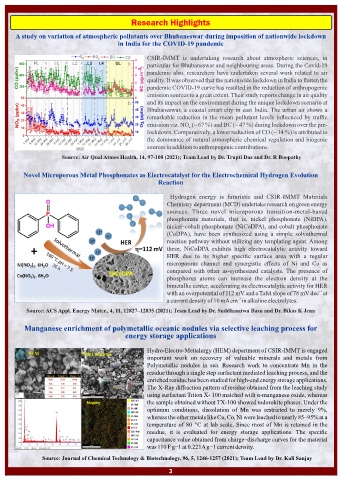
Novel Microporous Metal Phosphonates as Electrocatalyst for the Electrochemical Hydrogen Evolution
Reaction
Hydrogen energy is futuristic and CSIR-IMMT Materials
Chemistry department (MCD) undertake research on green energy
sources. Three novel microporous transition-metal-based
phosphonate materials, that is, nickel phosphonate (NiDPA),
nickel–cobalt phosphonate (NiCoDPA), and cobalt phosphonate
(CoDPA), have been synthesized using a simple solvothermal
reaction pathway without utilizing any templating agent. Among
these, NiCoDPA exhibits high electrocatalytic activity toward
HER due to its higher specific surface area with a regular
microporous channel and synergistic effects of Ni and Co as
compared with other as-synthesized catalysts. The presence of
phosphorus atoms can increase the electron density at the
bimetallic center, accelerating its electrocatalytic activity for HER
–1
with an overpotential of 112 mV and a Tafel slope of 78 mV dec at
–2
a current density of 10 mA cm in alkaline electrolytes.
Source: ACS Appl. Energy Mater., 4, 11, 12827–12835 (2021); Team Lead by Dr. Suddhasatwa Basu and Dr. Bikas K Jena
Manganese enrichment of polymetallic oceanic nodules via selective leaching process for
energy storage applications
Hydro-Electro-Mettalurgy (HEM) department of CSIR-IMMT is engaged
important work on recovery of valuable minerals and metals from
Polymetallic nodules in sea. Research work to concentrate Mn in the
residue through a single step surfactant mediated leaching process, and the
enriched residue has been studied for high-end energy storage applications.
The X-Ray diffraction pattern of residue obtained from the leaching study
using surfactant Triton X- 100 matched with α-manganese oxide, whereas
the sample obtained without TX-100 showed todorokite phases. Under the
optimum conditions, dissolution of Mn was restricted to merely 9%,
whereas the other metals like Cu, Co, Ni were leached to nearly 85–95% at a
temperature of 80 °C at lab scale. Since most of Mn is retained in the
residue, it is evaluated for energy storage applications. The specific
capacitance value obtained from charge–discharge curves for the material
was 110 F g−1 at 0.221A g−1 current density.
Source: Journal of Chemical Technology & Biotechnology, 96, 5, 1246-1257 (2021); Team Lead by Dr. Kali Sanjay
3